How To Repair Hair Cells In Ear
Introduction
Neural stem cells have the power to cocky-renew and differentiate into various types of nerve cells and take been used as a potential treatment for various diseases. Some supporting cells with proliferation power in the inner ear are also called sensory precursor cells (Monzack and Cunningham, 2022). Stem jail cell therapy refers to a treatment that uses the multifariousness of stalk cells to induce differentiation into the same construction equally the original shape and part when the normal construction of the organism is damaged or changed. This treatment can supplement the defective and damaged inner ear hair cells. The differentiation mechanism of inner ear stem cells is a complex regulatory network system. In add-on to the expression sequence of genes playing an important role in the regulation of their differentiation, the secretion and interaction of diverse cytokines are also closely related to it. Now, the molecular regulation mechanism of inner ear stalk cells is notwithstanding unclear. With the increase of related research and the development of molecular biology engineering science, the molecular regulation mechanism of neural stem jail cell differentiation has gradually been elucidated. In recent years, the inner ear sensory forerunner cells or stem cells are induced to re-enter the jail cell bike by activating the inner ear-related signal pathways, and proliferate and differentiate into pilus cells, and finally restore hearing or vestibular function, which take gradually become a research hotspot (Monzack and Cunningham, 2022). The proliferation and differentiation of sensory precursor cells are regulated by various related signal pathways, including WNT, Notch, BMP/Smad, FGF, IGF, and Shh signal pathways (Schimmang, 2007; Munnamalai and Fekete, 2022; Waqas et al., 2022; Wu et al., 2022). The regulation of these betoken pathways and related factors is very important for the induction of inner ear stem cells and sensory precursor cells to differentiate into mature inner ear pilus cells.
In contempo years, with the in-depth research on the pathogenesis of deafness, more and more researchers are trying to care for hearing diseases through stalk cell therapy. At nowadays, there are ii main ways to regenerate hair cells in the inner ear: one is that the supporting cells will re-enter the cell bicycle and differentiate into hair cells after mitosis. Some other way is that supporting cells will directly transdifferentiate into hair cells. In addition to Sox2+ cells, Lgr5+ cells, and Axin2+ cells that are frequently used for stem cell enquiry, Chai et al. (2012) found that the prison cell population of Fzd9+ cells is much smaller than Lgr5+ progenitor cells in the cochlea of newborn Fzd9-CreER/Rosa26-tdTomato mice (Zhang et al., 2022). They besides found that Fzd9+ cells accept the ability to generate hair cells in vivo. In addition, the proliferation, differentiation, and hair cell generation capabilities of Fzd9+ cells cultured in vitro are similar to those of Lgr5+ progenitor cells. Therefore, Fzd9+ cells may be the main functional progenitor cell type in Lgr5+ cells (Zhang et al., 2022). Therefore, Fzd9 can be used as a marker for hair jail cell progenitor, which is more specific than Lgr5 in hair cell progenitor.
Application of AAV Vectors in Inner Ear
The method of regulating the differentiation and evolution of stem cells through gene editing to promote the regeneration of inner ear hair cells has gradually become a research hotspot in the field of hearing. The ideal vector for gene editing needs to accurately deliver the nucleic acrid fragments into the inner ear and target cells and express them efficiently. In addition, the vector needs to have high transfection efficiency, controllable intensity and time, and high safety. Traditional viral vectors are toxic and acquit express gene capacity. In contempo years, researchers have discovered some ideal adeno-associated virus (AAV) vectors that can be transfected in the inner ear (Iizuka et al., 2008; Giannelli et al., 2022; Gu et al., 2022; Tan et al., 2022). AAV vector-mediated factor therapy has been canonical in the United States and can be used to treat rare hereditary eye diseases (Tan et al., 2022). Omar Akil et al. have used the AAV virus vector to evangelize the VGLUT3 gene to VGLUT3 knockout mice and achieved significant comeback in hearing (Akil et al., 2022). In order to avoid the shortcomings of low efficiency of traditional AAVs infecting outer hair cells, Zinn et al. made the vector AAV2/Anc80L65 based on the original sequence of the AAV capsid (Zinn et al., 2022). This vector tin can efficiently target the inner hair cells and outer pilus cells of the cochlea, which indicates an of import breakthrough in the research of AAV vectors in cochlear cell targeting. Kevin and colleagues found that AAV2.7m8 can also efficiently infect inner pilus cells and outer hair cells in the cochlea, and the efficiency of infecting outer hair cells is even college than that of Anc80L65 (Gu et al., 2022; Isgrig et al., 2022). In add-on, AAV2.7m8 not only preferentially targets cochlear hair cells, but it can too efficiently infect Lgr5+ supporting cells. Tan et al. constructed the AAV mutant AAV-inner ear (AAV-ie) by inserting the polypeptide DGTLAVPFK, which can increase the infection efficiency by generating a transmembrane construction (Tan et al., 2022). AAV-ie can efficiently infect cochlear hair cells and vestibular hair cells. In the cochlea, since GFAP protein is expressed in supporting cells only non in hair cells, the GFAP promoter tin can exist used to accomplish specific expression of AAV-ie genes in supporting cells. In addition, studies have found that AAV-ie does non touch the function of pilus cells and the auditory system (Tan et al., 2022).
Point Pathways Related to Hair Cell Regeneration
In recent years, researchers have discovered through the study of related pathways in the inner ear that WNTs, FGFs, BMP, Shh, Notch, and other signaling molecules play different roles in the process of hair cell regeneration (Figure 1). Therefore, hair cell regeneration does not depend on the activation of 1 betoken pathway but is coordinated by multiple signal pathways.
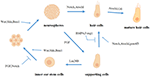
Figure 1. The function of diverse signal pathways and related factors in the process of pilus prison cell regeneration.
The Role of Wnt Signaling Pathway in Hair Cell Regeneration
The WNT/β-catenin signaling pathway plays an important regulatory role in cell proliferation, cell fate conclusion, and pilus prison cell differentiation (Jacques et al., 2022). When the WNT/β-catenin signal is inhibited, the proliferation ability of sensory cells is reduced, and the activation of the WNT/β-catenin bespeak promotes the increment of hair cells (Jacques et al., 2022; Shi et al., 2022). The target genes Lgr5 and Lgr6 of the WNT pathway are expressed in embryonic and neonatal cochlear progenitor cells (Chai et al., 2022; Samarajeewa et al., 2022). Lgr5+ cells have the characteristics of pilus cell progenitor cells, such every bit proliferation, self-renewal, and regeneration into hair cells (Chai et al., 2022; Shi et al., 2022; Cox et al., 2022; Wang et al., 2022). Later on WNT agonist handling, the proliferation power of the Lgr5+ progenitor cells of the neonatal mouse cochlea and their power to differentiate into pilus cells were enhanced (Romero-Carvajal et al., 2022; Samarajeewa et al., 2022). WNT agonists or overexpression of β-catenin can increment the proliferation of Lgr5+ progenitor cells and promote the formation of hair cells, while WNT antagonists inhibit the proliferation of Lgr5+ cells and the power to regenerate hair cells (Chai et al., 2022; Shi et al., 2022, 2022). In newborn mice, the activation of WNT signaling can also crusade Axin2-positive cells to proliferate and differentiate into hair cells and supporting cells (Jan et al., 2022). When the expression of β-catenin and Atoh1 are activated simultaneously, the proliferation power and the directed differentiation power of Lgr5+ cells into pilus cells and the survival ability of newly regenerated pilus cells are enhanced (Kuo et al., 2022; Mittal et al., 2022). In improver, the combination of WNT betoken activation and Notch signal inhibition in the cochlea of newborn mice has likewise been shown to promote the proliferation of supporting cells and the regeneration of hair cells (Ni et al., 2022).
The Role of Notch Signaling Pathway in Hair Cell Regeneration
The Notch signaling pathway plays an important role in the development and regeneration of hair cells. Supporting cells are induced to transdifferentiate into hair cells when Notch signaling in the cochlea of newborn mice is suppressed (Yamamoto et al., 2006; Mizutari et al., 2022; Bramhall et al., 2022). Researchers establish that activating the Notch pathway in supporting cells puts them in a quiescent country and inhibits pilus cell regeneration (Ma et al., 2008; Daudet et al., 2009). Previous studies plant that in the cochlea of neonatal mammals, the expression of Atoh1 was upwards-regulated after γ-secretase inhibitor blocked the Notch indicate, and caused the side by side supporting cells to transdifferentiate into hair cells. Still, this newly generated pilus cell does not accept the characteristics of a mature hair cell (Korrapati et al., 2022; Mizutari et al., 2022). In add-on, knocking out Hes1 and Hes5 members of the Hes family unit with siRNA can also upward-regulate the expression of Atoh1, leading to an increase in the number of hair cells in the cochlea of newborn and developed mice (Du et al., 2022). Provisional inhibition of Notch signal can advance Lgr5+ cells in supporting cells to become new hair cells (Mittal et al., 2022). Co-activating the jail cell wheel activator Myc and Notch1 in the inner ear can induce the proliferation of different types of cochlear sensory epithelial cells. After the cochlear supporting cells are reprogrammed by regulating the action of MYC/NICD, Atoh1 tin can induce the supporting cells in vivo and in vitro to effectively transdifferentiate into hair prison cell-like cells (Shu et al., 2022). Li et al. (2015) found that the combined inhibition of WNT and Notch signals can reduce the generation of hair cells, indicating that the progenitor cell proliferation miracle that occurs after Notch inhibition is mainly regulated by the WNT pathway (Li et al., 2022).
The Role of Hedgehog Signaling Pathway in Hair Cell Regeneration
The Hedgehog pathway is a highly conserved signaling pathway, which plays an important role in regulating the proliferation and cell fate determination and differentiation of progenitor cells in the early developmental phase of the inner ear (Zarei et al., 2022). Previous studies have institute that inactivation of Hedgehog signaling causes abnormal proliferation and differentiation of mammalian cochlear cells, leading to abnormal development of cochlear structure and office (Brown and Epstein, 2022; Bok et al., 2022; Son et al., 2022). Lu et al. (2013) found that after neomycin harm, Shh signaling may inhibit the role of pRb to promote sensory epithelial cells in the cochlea of newborn mice to re-enter the cell cycle and regenerate into new pilus cells. Chen et al. (2017) found that recombinant Shh protein can effectively promote the proliferation and differentiation of Lgr5+ progenitor cells in the cochlea of newborn mice. In addition, afterwards R26-SmoM2 mouse cochlear explants are treated with neomycin, Hedgehog signal activation can significantly promote cochlear epithelial cell proliferation and pilus cell regeneration (Chen et al., 2022).
The Role of FGF Signaling Pathway in Pilus Cell Regeneration
The FGF signaling pathway activates the gene regulatory network of the early development of the inner ear and induces the germination of the pre-placodal region and the auditory placode (Padanad et al., 2022; Ornitz and Itoh, 2022). In addition, the FGF signaling pathway plays a central part in the spontaneous regeneration of lower vertebrate hair cells and the induction and differentiation of stalk cells into pilus cells. Ku et al. plant that when the chicken utricle was damaged by ototoxic drugs, the decrease of FGF expression level promoted the proliferation of supporting cells (Ku et al., 2022). Jiang et al. found that the inhibition of the FGF and Notch pathway in the process of supporting cell proliferation is consistent with the downwards-regulation of the cycl-dependent protein kinase (CDK) inhibitor CDKN1b (p27/kip1; Jiang et al., 2022). Therefore, the inhibition of FGF and Notch signaling pathway is the triggering gene for supporting prison cell proliferation. Piotrowski et al. establish through scRNA-seq that the deletion of Fgf3 expression in zebrafish supporting cells resulted in enhanced hair cell regeneration and increased the number of cells in neuromast (Lush et al., 2022). Lee et al. (2016) used drugs and genes to block FGF signaling and found that inhibiting FGF signaling can significantly inhibit the regeneration of hair cells in neuromast. Tang et al. institute that the FGF signaling pathway and WNT signaling pathway have a coordinated regulatory upshot on the proliferation of zebrafish lateral line cells (Tang et al., 2022). When the FGF point is inhibited, the WNT pathway-mediated cell proliferation will also be inhibited. In the absence of WNT activity, activating the FGF bespeak with bFGF volition restore part of supporting jail cell proliferation and hair cell regeneration.
Transcription Factors Related to Hair Prison cell Regeneration
Atoh1 is a transcription gene with a helix-loop-helix construction, which is essential for the differentiation of hair cells during the evolution of the inner ear (Bermingham et al., 1999). In animal experiments, it was found that the sensory epithelium of the cochlea of Atoh1 knockout mice differentiated into supporting cells, only not cochlear hair cells and vestibular pilus cells (Chen et al., 2002). Therefore, Atoh1 gene is an essential transcription gene in the procedure of hair cell generation. In in vitro experiments, researchers found that activating the expression of Atoh1 in the cochlea or vestibular sensory epithelium can induce hair prison cell regeneration (Bermingham et al., 1999; Izumikawa et al., 2005; Gubbels et al., 2008; Liu et al., 2022). In damaged mouse utricle, overexpression of Atoh1 can induce the proliferation of supporting cells and promote them transdifferentiate into pilus prison cell-like cells (Jen et al., 2022). In addition, the overexpression of Atoh1 also caused the upwards-regulation of many hair prison cell-related genes just did not include the hair cell maturation-related genes. Cheng found that the expression of Atoh1 in prenatal rats can induce supporting cells to differentiate into sensory hair cells, merely the regenerated hair cells are immature, which may exist due to the absence of Atoh1 downwardly-regulation during hair cell development (Cheng, 2022). Therefore, pilus prison cell regeneration and maturation are non merely regulated by Atoh1, simply too coordinated by other transcription factors.
Gfi1 is ane of the GPS (Gfi1/pag3/SENS) family transcription factors with a zinc finger domain. During the development of the inner ear, Gfi1 plays an important function in the normal differentiation, survival, and maturation of hair cells (Wallis et al., 2003). Gfi1 expression-deficient mice showed aberrant hair cell evolution, such every bit cochlear outer pilus cells and inner hair cells degenerating from the basal plow to the apex plow until they were completely absent (Wallis et al., 2003; Li and Doetzlhofer, 2022). Matern et al. found that when Gfi1 expression is absent, the maturation of hair cells in the cochlea and vestibule is blocked, and the expression of hair cell maturation markers, such as Strc, Tmc1, and Ocm, is inhibited (Matern et al., 2022). Lee et al. constitute that Gfi1 and upstream Atoh1 coordinately regulate the regeneration of cochlear hair cells in newborn mice (Lee et al., 2022).
The secreted protein os morphogenetic protein 4 (BMP4) tin regulate inner ear morphogenesis and hair cell development. Lewis et al. found that when the cochlea cultured in vitro is damaged, the addition of BMP4 can inhibit the expression of Atoh1 in supporting cells, thereby inhibiting supporting cell mitosis and hair jail cell regeneration (Lewis et al., 2022). Later treatment with the BMP4 inhibitor noggin, the number of regenerated pilus cells increased (Lewis et al., 2022).
Bmi1 is a member of the Polycomb protein family and plays a regulatory part in the proliferation of progenitor cells and stem cells in multiple organs (Bruggeman et al., 2007; López-Arribillaga et al., 2022; Lu et al., 2022). Recently, researchers have discovered that Bmi1 activates WNT signaling by inhibiting the Dickkopf (DKK) family with WNT inhibitor functions (Cho et al., 2022). Lu et al. (2017) demonstrated in in vitro experiments that knocking out Bmi1 tin significantly inhibit the proliferation of Lgr5+ progenitor cells in the cochlea of neonatal mice afterwards neomycin impairment. Compared with wild-type mice, the expression of DKK1 in Bmi1−/− neonatal mice was significantly up-regulated, while the expression of β-catenin and Lgr5 was significantly down-regulated. In addition, the WNT agonist BIO inhibited the decline of the proliferation ability of Bmi1−/− mouse supporting cells, indicating that Bmi1 affects the proliferation of supporting cells and Lgr5+ progenitor cells through the regulation of WNT signaling pathway. Therefore, Bmi1 may exist a new therapeutic target for pilus cell regeneration.
The POU domain transcription factor Pou4f3 plays an important role in the development of inner ear hair cells (Masuda et al., 2022). Recent studies have found that in the developed mouse cochlea, the ectopic expression of Pou4f3 tin can promote the transdifferentiation of supporting cells into pilus cells together with Atoh1 (Walters et al., 2022). The activation of downstream target genes by Pou4f3 can promote Atoh1-mediated pilus cell evolution and survival (Walters et al., 2022). Pou4f3 not only regulates the expression of Gfi1 and Nr2f2 related to hair cell regeneration (Zhong et al., 2022) only too is the direct target gene of Atoh1 during pilus cell development (Lee et al., 2022). Atoh1-Pou4f3-target gene (such every bit Gfi1) is not just an important molecular pathway for regulating the fate and differentiation of hair cells but also an important molecular pathway for hair cell maturation and survival. Therefore, POU4f3 every bit a therapeutic target, activating its action alone or coordinating with Atoh1 may promote the regeneration of auditory hair cells.
Foxg1 is one of the forkhead box genes involved in morphogenesis, prison cell fate determination, and proliferation. Previous studies reported that Foxg1 is necessary for the morphogenesis of the inner ear in mammals (Pauley et al., 2006; Hwang et al., 2009; He et al., 2022). Pauley et al. found that in Foxg1 knockout mice, the cochlear duct became shorter, the number of hair cells increased, the vestibule shrank, the growth of axons, and the distribution of vestibular neurons were abnormal (Pauley et al., 2006). Chai et al. (2012) found that conditionally knocking out Foxg1 in Sox2+ supporting cells and Lgr5+ progenitor cells of newborn mice induced these cells to transdifferentiate into pilus cells, resulting in a significant increase in the number of hair cells and a significant decrease in supporting cells (Zhang et al., 2022). In improver, they also found that knocking out Foxg1 expression downwardly-regulated the expression of several Notch signaling pathway factors such every bit Hes1, Hes5, and Hey1. At the aforementioned time, the expression of cell cycle-dependent kinase (Cdk2) was likewise downwardly-regulated, while the expression of cell bike repressors Cdkn1c, Cdkn2a, and Gadd45g were upwardly-regulated, indicating that Foxg1 knockout may cause jail cell wheel and Notch signaling pathway to be inhibited, which led to the increment of pilus cells (Zhang et al., 2022). Chai et al. (2012) also found that knocking out Foxg1 in Sox9+ supporting cells can promote the transdifferentiation of supporting cells into hair cells in neonatal mouse utricle (Zhang et al., 2022). The in a higher place studies provide evidence for the office of Foxg1 in the regeneration of cochlear hair cells in newborn mice.
The Lin28 cistron encodes an evolutionarily highly conserved RNA binding protein, which is known to regulate the larval development time of Caenorhabditis elegans (Moss and Tang, 2003). In humans and mice, Lin28a and its homolog Lin28b are primal regulators of organ growth, metabolism, tumorigenesis, and tissue repair (Ambros and Horvitz, 1984; Shyh-Chang and Daley, 2022). Doetzlhofer and colleagues found that the pathway composed of Lin28b and let-7 miRNAs regulates the generation of new pilus cells in P2 mouse cochlear explants (Golden et al., 2022). The function of Lin28b is mainly to promote the generation of new hair cells, while the function of permit-7 miRNAs is to inhibit the generation of new hair cells. Lin28b functional defect or overexpression of permit-7g miRNAs leads to the inhibition of the activity of Akt-mTOR complex one (mTORC1) so that immature supporting cells cannot be transdifferentiated into hair cells (Li and Doetzlhofer, 2022). On the contrary, overexpression of Lin28b increased the activity of Akt-mTORC1, and dedifferentiated the maturing supporting cells into progenitor-like cells, and generated hair cells through mitotic and non-mitotic mechanisms (Li and Doetzlhofer, 2022). These findings may provide new strategies for hereafter hair cell regeneration treatments.
In add-on, Menendez et al. (2020) plant that the combination of 4 transcription factors (Six1, Atoh1, Pou4f3, and Gfi1) can transdifferentiate neonatal mouse supporting cells (P8), mouse embryonic fibroblasts, and developed mouse tail fibroblasts into induced hair cells (IHCs). IHCs accept various characteristics of pilus cells, such every bit morphology, transcriptome and epigenetic characteristics, electrophysiological characteristics, mechanosensory channel expression, and ototoxin susceptibility. Therefore, IHCs can exist used as an ideal in vitro model for studying hair jail cell part, maturation, regeneration, and ototoxin sensitivity.
The Function of Epigenetic Regulation of Hair Cell Regeneration
Epigenetic modification plays an important role in the development of the inner ear, and recent studies have found that it also has an important regulatory role in hair cell regeneration. During the development of zebrafish larvae, the inhibition of LSD1 by 2-PCPA decreased the expression of WNT/β-catenin and FGF signaling pathways, thereby significantly inhibiting the regeneration of supporting cells and hair cells subsequently neomycin damage (He et al., 2022). The inhibition of G9a/GLP by BIX01294 significantly reduced the dimethylation of H3K9 in the zebrafish lateral line (Tang et al., 2022). The defect of H3K9me2 significantly inhibited the WNT/β-catenin and FGF signaling pathways, which significantly reduced the proliferation of supporting cells after neomycin harm, and ultimately lead to the reduction of mitotic regeneration of hair cells in the zebrafish lateral line (Tang et al., 2022). Previous studies have found that when five-azacytidine, a DNA methyltransferase (Dnmt) inhibitor, is injected into the cochlea of mature mice that are chemically deafened, Dna demethylation may promote the regeneration of hair cells in the cochlea of mature mice (Deng and Hu, 2022). The reward of this epigenetic method is that the DNA sequence remains unchanged during the process without integrating the exogenous Dna sequence.
Conclusion
At that place are various means and mechanisms that cause sensorineural hearing loss, among which irreversible impairment to inner ear hair cells is the chief crusade of sensorineural hearing loss. Although the commonly used hearing aids and cochlear implants in clinical do also better the hearing of patients, their result depends on the quantity and quality of residual pilus cells and spiral neurons. Therefore, the ideal way to treat sensorineural hearing loss is to regenerate hair cells through stem cells to repair the structure and function of the cochlea so as to fundamentally restore hearing. Stem jail cell therapy in the auditory field has been a enquiry hotspot in recent years. Although some progress has been made, almost all are results at the animal level, and there is withal a long style to go before clinical transformation. The microenvironment of inner ear stalk cells and the interaction with neighboring cells are very important for inner ear stem cells or sensory precursor cells to induce differentiation into mature inner ear hair cells. In the reported studies, the efficiency of differentiation of inner ear stem cells or sensory forerunner cells into hair cells is still low. An insufficient number of new hair cells, young new hair cells without the role of mature hair cells, and long–term survival of new hair cells are all central problems and difficulties that need to exist solved urgently. All these indicate that it is more difficult to regulate a single signal pathway to regenerate functional pilus cells, and it may require coordinated regulation of multiple genes to finer promote hair jail cell regeneration and the functional maturity and survival of new pilus cells. At present, inducing the committed differentiation of stem cells into hair cells or nervus cells, the exploration of the methods of stalk jail cell transplantation into the inner ear, and the condom research of stem jail cell transplantation have laid the foundation for the transplantation of stem cells in vivo.
Author Contributions
SX and NY wrote the article. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the enquiry was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Notation
All claims expressed in this commodity are solely those of the authors and exercise not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any production that may exist evaluated in this article, or claim that may be made past its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AAV, adeno-associated virus; AAV-ie, AAV-inner ear; CDK, cycl-dependent poly peptide kinase; BMP4, os morphogenetic protein 4; DKK, Dickkopf; mTORC1, Akt-mTOR complex i; Dnmt, DNA methyltransferase.
References
Akil, O., Seal, R. P., Burke, Grand., Wang, C., Alemi, A., During, G., et al. (2012). Restoration of hearing in the VGLUT3 knockout mouse using virally mediated gene therapy. Neuron 75, 283–293. doi: 10.1016/j.neuron.2012.05.019
PubMed Abstract | CrossRef Full Text | Google Scholar
Bermingham, N. A., Hassan, B. A., Price, S. D., Vollrath, M. A., Ben-Arie, N., Eatock, R. A., et al. (1999). Math1: an essential gene for the generation of inner ear pilus cells. Science 284, 1837–1841. doi: 10.1126/science.284.5421.1837
PubMed Abstract | CrossRef Full Text | Google Scholar
Bok, J., Zenczak, C., Hwang, C. H., and Wu, D. One thousand. (2013). Auditory ganglion source of Sonic hedgehog regulates timing of cell cycle leave and differentiation of mammalian cochlear hair cells. Proc. Natl. Acad. Sci. U S A 110, 13869–13874. doi: 10.1073/pnas.1222341110
PubMed Abstruse | CrossRef Total Text | Google Scholar
Bramhall, N. F., Shi, F., Arnold, K., Hochedlinger, K., and Edge, A. South. B. (2014). Lgr5-positive supporting cells generate new hair cells in the postnatal cochlea. Stalk Cell Rep. 2, 311–322. doi: 10.1016/j.stemcr.2014.01.008
PubMed Abstract | CrossRef Full Text | Google Scholar
Brown, A. S., and Epstein, D. J. (2011). Otic ablation of smoothened reveals direct and indirect requirements for Hedgehog signaling in inner ear development. Development (Cambridge, England) 138, 3967–3976. doi: 10.1242/dev.066126
PubMed Abstruse | CrossRef Full Text | Google Scholar
Bruggeman, S. W. M., Hulsman, D., Tanger, East., Buckle, T., Blom, M., Zevenhoven, J., et al. (2007). Bmi1 controls tumor evolution in an Ink4a/Arf-contained way in a mouse model for glioma. Cancer Cell 12, 328–341. doi: x.1016/j.ccr.2007.08.032
PubMed Abstract | CrossRef Total Text | Google Scholar
Chai, R., Kuo, B., Wang, T., Liaw, E. J., Xia, A., January, T. A., et al. (2012). Wnt signaling induces proliferation of sensory precursors in the postnatal mouse cochlea. Proc. Natl. Acad. Sci. U S A 109, 8167–8172. doi: 10.1073/pnas.1202774109
PubMed Abstract | CrossRef Full Text | Google Scholar
Chai, R., Xia, A., Wang, T., Jan, T. A., Hayashi, T., Bermingham-McDonogh, O., et al. (2011). Dynamic expression of Lgr5, a Wnt target cistron, in the developing and mature mouse cochlea. J. Assoc. Res. Otolaryngol. 12, 455–469. doi: 10.1007/s10162-011-0267-2
PubMed Abstract | CrossRef Full Text | Google Scholar
Chen, P., Johnson, J. E., Zoghbi, H. Y., and Segil, N. (2002). The role of Math1 in inner ear evolution: uncoupling the institution of the sensory primordium from hair cell fate decision. Development 129, 2495–2505. doi: 10.3410/f.1006295.78812
PubMed Abstract | CrossRef Full Text | Google Scholar
Chen, Y., Lu, X., Guo, Fifty., Ni, Westward., Zhang, Y., Zhao, L., et al. (2017). Hedgehog signaling promotes the proliferation and subsequent pilus cell formation of progenitor cells in the neonatal mouse cochlea. Front. Mol. Neurosci. 10:426. doi: x.3389/fnmol.2017.00426
PubMed Abstruse | CrossRef Full Text | Google Scholar
Cho, J.-H., Dimri, M., and Dimri, G. P. (2013). A positive feedback loop regulates the expression of polycomb group protein BMI1 via WNT signaling pathway. J. Biol. Chem. 288, 3406–3418. doi: 10.1074/jbc.M112.422931
PubMed Abstruse | CrossRef Full Text | Google Scholar
Cox, B. C., Chai, R., Lenoir, A., Liu, Z., Zhang, L., Nguyen, D.-H., et al. (2014). Spontaneous hair prison cell regeneration in the neonatal mouse cochlea in vivo. Development 141, 816–829. doi: 10.1242/dev.103036
PubMed Abstract | CrossRef Full Text | Google Scholar
Daudet, N., Gibson, R., Shang, J., Bernard, A., Lewis, J., and Stone, J. (2009). Notch regulation of progenitor jail cell behavior in quiescent and regenerating auditory epithelium of mature birds. Dev. Biol. 326, 86–100. doi: 10.1016/j.ydbio.2008.10.033
PubMed Abstract | CrossRef Total Text | Google Scholar
Du, X., Cai, Q., Westward, M. B., Youm, I., Huang, X., Li, Westward., et al. (2018). Regeneration of cochlear hair cells and hearing recovery through Hes1 modulation with siRNA nanoparticles in adult guinea pigs. Mol. Ther. 26, 1313–1326. doi: ten.1016/j.ymthe.2018.03.004
PubMed Abstract | CrossRef Full Text | Google Scholar
Giannelli, S. G., Luoni, Grand., Castoldi, 5., Massimino, 50., Cabassi, T., Angeloni, D., et al. (2018). Cas9/sgRNA selective targeting of the P23H Rhodopsin mutant allele for treating retinitis pigmentosa by intravitreal AAV9.PHP.B-based commitment. Hum. Mol. Genet. 27, 761–779. doi: 10.1093/hmg/ddx438
PubMed Abstract | CrossRef Full Text | Google Scholar
Golden, E. J., Benito-Gonzalez, A., and Doetzlhofer, A. (2015). The RNA-binding poly peptide LIN28B regulates developmental timing in the mammalian cochlea. Proc. Natl. Acad. Sci. U Southward A 112, E3864–E3873. doi: 10.1073/pnas.1501077112
PubMed Abstract | CrossRef Total Text | Google Scholar
Gu, Ten., Chai, R., Guo, L., Dong, B., Li, W., Shu, Y., et al. (2019). Transduction of adeno-associated virus vectors targeting pilus cells and supporting cells in the neonatal mouse cochlea. Front end. Cell. Neurosci. 13:8. doi: x.3389/fncel.2019.00008
PubMed Abstract | CrossRef Full Text | Google Scholar
Gubbels, S. P., Woessner, D. W., Mitchell, J. C., Ricci, A. J., and Brigande, J. 5. (2008). Functional auditory hair cells produced in the mammalian cochlea by in utero gene transfer. Nature 455, 537–541. doi: ten.1038/nature07265
PubMed Abstract | CrossRef Total Text | Google Scholar
He, Y., Tang, D., Cai, C., Chai, R., and Li, H. (2016). LSD1 is required for hair cell regeneration in zebrafish. Mol. Neurobiol. 53, 2421–2434. doi: 10.1007/s12035-015-9206-2
PubMed Abstract | CrossRef Total Text | Google Scholar
He, Z., Fang, Q., Li, H., Buwei, S., Zhang, Y., Zhang, Y., et al. (2018). The role of FOXG1 in the postnatal evolution and survival of mouse cochlear hair cells. Neuropharmacology 144, 43–57. doi: 10.1016/j.neuropharm.2018.10.021
PubMed Abstract | CrossRef Full Text | Google Scholar
Hwang, C. H., Simeone, A., Lai, Due east., and Wu, D. Grand. (2009). Foxg1 is required for proper separation and formation of sensory cristae during inner ear development. Dev. Dyn. 238, 2725–2734. doi: 10.1002/dvdy.22111
PubMed Abstract | CrossRef Full Text | Google Scholar
Iizuka, T., Kanzaki, Southward., Mochizuki, H., Inoshita, A., Narui, Y., Furukawa, K., et al. (2008). Noninvasive in vivo delivery of transgene via adeno-associated virus into supporting cells of the neonatal mouse cochlea. Hum. Gene Ther. 19, 384–390. doi: ten.1089/hum.2007.167
PubMed Abstruse | CrossRef Full Text | Google Scholar
Isgrig, Thousand., McDougald, D. S., Zhu, J., Wang, H. J., Bennett, J., and Chien, W. W. (2019). AAV2.7m8 is a powerful viral vector for inner ear gene therapy. Nat. Commun. 10:427. doi: 10.1038/s41467-018-08243-1
PubMed Abstract | CrossRef Full Text | Google Scholar
Izumikawa, Thousand. R., Minoda, K., Kawamoto, Thou. A., Abrashkin, D. L., Swiderski, D. F., Dolan, D. East., et al. (2005). Auditory pilus cell replacement and hearing comeback past Atoh1 gene therapy in deafened mammals. Nat. Med. 11, 271–276. doi: 10.1038/nm1193
PubMed Abstract | CrossRef Full Text | Google Scholar
Jacques, B. E., Montgomery Four, West. H., Uribe, P. M., Yatteau, A., Asuncion, J. D., Resendiz, G., et al. (2014). The role of Wnt/β-catenin signaling in proliferation and regeneration of the developing basilar papilla and lateral line. Dev. Neurobiol. 74, 438–456. doi: x.1002/dneu.22134
PubMed Abstract | CrossRef Total Text | Google Scholar
Jacques, B. Due east., Puligilla, C., Weichert, R. M., Ferrer-Vaquer, A., Hadjantonakis, A.-K., Kelley, Chiliad. W., et al. (2012). A dual function for canonical Wnt/β-catenin signaling in the developing mammalian cochlea. Evolution 139, 4395–4404. doi: 10.1242/dev.080358
PubMed Abstract | CrossRef Full Text | Google Scholar
January, T. A., Chai, R., Sayyid, Z. Northward., van Amerongen, R., Xia, A., Wang, T., et al. (2013). Tympanic edge cells are Wnt-responsive and tin act every bit progenitors for postnatal mouse cochlear cells. Development 140, 1196–1206. doi: 10.1242/dev.087528
PubMed Abstract | CrossRef Full Text | Google Scholar
Jen, H.-I., Hill, M. C., Tao, Fifty., Sheng, K., Cao, W., Zhang, H., et al. (2019). Transcriptomic and epigenetic regulation of pilus cell regeneration in the mouse utricle and its potentiation by Atoh1. eLife 8:e44328. doi: 10.7554/eLife.44328
PubMed Abstract | CrossRef Full Text | Google Scholar
Jiang, L., Romero-Carvajal, A., Haug, J. S., Seidel, C. West., and Piotrowski, T. (2014). Gene-expression assay of hair cell regeneration in the zebrafish lateral line. Proc. Natl. Acad. Sci. U S A 111, E1383–E1392. doi: 10.1073/pnas.1402898111
PubMed Abstruse | CrossRef Full Text | Google Scholar
Korrapati, South., Roux, I., Glowatzki, E., and Doetzlhofer, A. (2013). Notch signaling limits supporting cell plasticity in the pilus cell-damaged early postnatal murine cochlea. PLoS One 8:e73276. doi: ten.1371/journal.pone.0073276
PubMed Abstract | CrossRef Total Text | Google Scholar
Ku, Y.-C., Renaud, North. A., Veile, R. A., Helms, C., Voelker, C. C. J., Warchol, M. E., et al. (2014). The transcriptome of utricle hair cell regeneration in the avian inner ear. J. Neurosci. 34, 3523–3535. doi: 10.1523/JNEUROSCI.2606-thirteen.2014
PubMed Abstract | CrossRef Total Text | Google Scholar
Kuo, B. R., Baldwin, Due east. M., Layman, W. S., Taketo, M. Grand., and Zuo, J. (2015). In vivo cochlear hair jail cell generation and survival by coactivation of β-catenin and Atoh1. J. Neurosci. 35, 10786–10798. doi: 10.1523/JNEUROSCI.0967-15.2015
PubMed Abstract | CrossRef Full Text | Google Scholar
López-Arribillaga, E., Rodilla, V., Pellegrinet, L., Guiu, J., Iglesias, M., Roman, A. C., et al. (2015). Bmi1 regulates murine abdominal stem cell proliferation and self-renewal downstream of Notch. Evolution 142, 41–fifty. doi: ten.1242/dev.107714
PubMed Abstract | CrossRef Full Text | Google Scholar
Lee, S. G., Huang, Chiliad., Obholzer, N. D., Sun, Southward., Li, West., Petrillo, Thou., et al. (2016). Myc and Fgf are required for zebrafish neuromast hair cell regeneration. PLoS Ane 11:e0157768. doi: 10.1371/journal.pone.0157768
PubMed Abstract | CrossRef Full Text | Google Scholar
Lee, South., Song, J. -J., Beyer, L. A., Swiderski, D. Fifty., Prieskorn, D. G., Acar, M., et al. (2020). Combinatorial Atoh1 and Gfi1 induction enhances pilus cell regeneration in the adult cochlea. Sci. Rep. 10:21397. doi: 10.1038/s41598-020-78167-8
PubMed Abstract | CrossRef Full Text | Google Scholar
Lewis, R. M., Keller, J. J., Wan, 50., and Rock, J. Southward. (2018). Bone morphogenetic protein iv antagonizes hair prison cell regeneration in the avian auditory epithelium. Hear. Res. 364, 1–11. doi: 10.1016/j.heares.2018.04.008
PubMed Abstract | CrossRef Full Text | Google Scholar
Li, W., Wu, J., Yang, J., Sunday, S., Chai, R., Chen, Z.-Y., et al. (2015). Notch inhibition induces mitotically generated hair cells in mammalian cochleae via activating the Wnt pathway. Proc. Natl. Acad. Sci. U S A 112, 166–171. doi: ten.1073/pnas.1415901112
PubMed Abstract | CrossRef Full Text | Google Scholar
Li, X.-J., and Doetzlhofer, A. (2020). LIN28B/let-vii command the ability of neonatal murine auditory supporting cells to generate hair cells through mTOR signaling. Proc. Natl. Acad. Sci. U S A 117, 22225–22236. doi: x.1073/pnas.2000417117
PubMed Abstract | CrossRef Total Text | Google Scholar
Liu, Z., Dearman, J. A., Cox, B. C., Walters, B. J., Zhang, L., Ayrault, O., et al. (2012). Age-dependent in vivo conversion of mouse cochlear pillar and Deiters' cells to immature hair cells past Atoh1 ectopic expression. J. Neurosci. 32, 6600–6610. doi: x.1523/JNEUROSCI.0818-12.2012
PubMed Abstract | CrossRef Total Text | Google Scholar
Lu, Northward., Chen, Y., Wang, Z., Chen, Grand., Lin, Q., Chen, Z.-Y., et al. (2013). Sonic hedgehog initiates cochlear hair prison cell regeneration through downregulation of retinoblastoma protein. Biochem. Biophys. Res. Commun. 430, 700–705. doi: 10.1016/j.bbrc.2012.11.088
PubMed Abstract | CrossRef Full Text | Google Scholar
Lu, X., Lord's day, S., Qi, J., Li, Westward., Liu, L., Zhang, Y., et al. (2017). Bmi1 regulates the proliferation of cochlear supporting cells via the canonical Wnt signaling pathway. Mol. Neurobiol. 54, 1326–1339. doi: 10.1007/s12035-016-9686-eight
PubMed Abstruse | CrossRef Full Text | Google Scholar
Lush, K. East., Diaz, D. C., Koenecke, N., Baek, South., Boldt, H., St Peter, M. K., et al. (2019). scRNA-Seq reveals distinct stem cell populations that drive pilus cell regeneration after loss of Fgf and Notch signaling. eLife 8:e44431. doi: 10.7554/eLife.44431
PubMed Abstract | CrossRef Total Text | Google Scholar
Ma, E. Y., Rubel, E. W., and Raible, D. Westward. (2008). Notch signaling regulates the extent of hair jail cell regeneration in the zebrafish lateral line. J. Neurosci. 28, 2261–2273. doi: 10.1523/JNEUROSCI.4372-07.2008
PubMed Abstract | CrossRef Full Text | Google Scholar
Masuda, M., Dulon, D., Pak, Chiliad., Mullen, Fifty. M., Li, Y., Erkman, L., et al. (2011). Regulation of POU4F3 gene expression in hair cells past 5′ DNA in mice. Neuroscience 197, 48–64. doi: 10.1016/j.neuroscience.2011.09.033
PubMed Abstruse | CrossRef Total Text | Google Scholar
Matern, Chiliad. South., Milon, B., Lipford, Due east. L., McMurray, Chiliad., Ogawa, Y., Tkaczuk, A., et al. (2020). GFI1 functions to repress neuronal gene expression in the developing inner ear hair cells. Development 147:dev186015. doi: ten.1242/dev.186015
PubMed Abstruse | CrossRef Full Text | Google Scholar
Menendez, Fifty., Trecek, T., Gopalakrishnan, Due south., Tao, L., Markowitz, A. 50., Yu, H. 5., et al. (2020). Generation of inner ear hair cells by direct lineage conversion of main somatic cells. eLife ix:e55249. doi: x.7554/eLife.55249
PubMed Abstract | CrossRef Full Text | Google Scholar
Mittal, R., Nguyen, D., Patel, A. P., Debs, L. H., Mittal, J., Yan, D., et al. (2017). Recent advancements in the regeneration of auditory hair cells and hearing restoration. Front. Mol. Neurosci. 10:236. doi: 10.3389/fnmol.2017.00236
PubMed Abstract | CrossRef Full Text | Google Scholar
Mizutari, K., Fujioka, K., Hosoya, M., Bramhall, N., Okano, H. J., Okano, H., et al. (2013). Notch inhibition induces cochlear hair cell regeneration and recovery of hearing after audio-visual trauma. Neuron 77, 58–69. doi: 10.1016/j.neuron.2012.x.032
PubMed Abstract | CrossRef Full Text | Google Scholar
Monzack, Eastward. Fifty., and Cunningham, L. L. (2013). Lead roles for supporting actors: disquisitional functions of inner ear supporting cells. Hear. Res. 303, 20–29. doi: 10.1016/j.heares.2013.01.008
PubMed Abstruse | CrossRef Full Text | Google Scholar
Moss, E. Grand., and Tang, L. (2003). Conservation of the heterochronic regulator Lin-28, its developmental expression and microRNA complementary sites. Dev. Biol. 258, 432–442. doi: ten.1016/s0012-1606(03)00126-ten
PubMed Abstract | CrossRef Full Text | Google Scholar
Munnamalai, 5., and Fekete, D. M. (2016). Notch-Wnt-Bmp crosstalk regulates radial patterning in the mouse cochlea in a spatiotemporal style. Development 143, 4003–4015. doi: ten.1242/dev.139469
PubMed Abstract | CrossRef Total Text | Google Scholar
Ni, W., Lin, C., Guo, Fifty., Wu, J., Chen, Y., Chai, R., et al. (2016). Extensive supporting cell proliferation and mitotic pilus cell generation by in vivo genetic reprogramming in the neonatal mouse cochlea. J. Neurosci. 36, 8734–8745. doi: 10.1523/JNEUROSCI.0060-xvi.2016
PubMed Abstruse | CrossRef Full Text | Google Scholar
Padanad, K. S., Bhat, N., Guo, B., and Riley, B. B. (2012). Conditions that influence the response to Fgf during otic placode induction. Dev. Biol. 364, 1–ten. doi: x.1016/j.ydbio.2012.01.022
PubMed Abstruse | CrossRef Total Text | Google Scholar
Romero-Carvajal, A., Acedo, J. N., Jiang, L., Kozlovskaja-Gumbrienė, A., Alexander, R., Li, H., et al. (2015). Regeneration of sensory hair cells requires localized interactions between the Notch and Wnt pathways. Dev. Prison cell 34, 267–282. doi: 10.1016/j.devcel.2015.05.025
PubMed Abstract | CrossRef Full Text | Google Scholar
Samarajeewa, A., Jacques, B. E., and Dabdoub, A. (2019). Therapeutic potential of wnt and notch signaling and epigenetic regulation in mammalian sensory hair cell regeneration. Mol. Ther. 27, 904–911. doi: 10.1016/j.ymthe.2019.03.017
PubMed Abstract | CrossRef Full Text | Google Scholar
Shi, F., Hu, L., and Border, A. Due south. B. (2013). Generation of hair cells in neonatal mice past β-catenin overexpression in Lgr5-positive cochlear progenitors. Proc. Nat. Acad. Sci. U. Southward. A. 110, 13851–13856. doi: 10.1073/pnas.1219952110
PubMed Abstract | CrossRef Full Text | Google Scholar
Shi, F., Hu, 50., Jacques, B. East., Mulvaney, J. F., Dabdoub, A., and Edge, A. Due south. B. (2014). β-Catenin is required for hair-cell differentiation in the cochlea. J. Neurosci. 34, 6470–6479. doi: 10.1523/JNEUROSCI.4305-xiii.2014
PubMed Abstruse | CrossRef Full Text | Google Scholar
Shi, F., Kempfle, J. South., and Edge, A. S. B. (2012). Wnt-responsive Lgr5-expressing stem cells are hair cell progenitors in the cochlea. J. Neurosci. 32, 9639–9648. doi: 10.1523/JNEUROSCI.1064-12.2012
PubMed Abstract | CrossRef Full Text | Google Scholar
Shu, Y., Li, W., Huang, Grand., Quan, Y. -Z., Scheffer, D., Tian, C., et al. (2019). Renewed proliferation in developed mouse cochlea and regeneration of hair cells. Nat. Commun. 10:5530. doi: x.1038/s41467-019-13157-7
PubMed Abstruse | CrossRef Full Text | Google Scholar
Son, E. J., Ma, J.-H., Ankamreddy, H., Shin, J.-O., Choi, J. Y., Wu, D. K., et al. (2015). Conserved function of sonic Hedgehog in tonotopic organization of the avian basilar papilla and mammalian cochlea. Proc. Nat. Acad. Sci. U S A 112, 3746–3751. doi: ten.1073/pnas.1417856112
PubMed Abstruse | CrossRef Full Text | Google Scholar
Tan, F., Chu, C., Qi, J., Li, W., You, D., Li, K., et al. (2019). AAV-ie enables safe and efficient gene transfer to inner ear cells. Nat. Commun. 10:3733. doi: 10.1038/s41467-019-11687-8
PubMed Abstract | CrossRef Total Text | Google Scholar
Tang, D., He, Y., Li, West., and Li, H. (2019). Wnt/β-catenin interacts with the FGF pathway to promote proliferation and regenerative cell proliferation in the zebrafish lateral line neuromast. Exp. Mol. Med. 51, 1–sixteen. doi: 10.1038/s12276-019-0247-ten.
CrossRef Full Text | Google Scholar
Tang, D., Lin, Q., He, Y., Chai, R., and Li, H. (2016). Inhibition of H3K9me2 reduces pilus cell regeneration after pilus jail cell loss in the zebrafish lateral line by down-regulating the Wnt and Fgf signaling pathways. Front. Mol. Neurosci. 9:39. doi: 10.3389/fnmol.2016.00039
PubMed Abstract | CrossRef Full Text | Google Scholar
Wallis, D., Hamblen, M., Zhou, Y., Venken, K. J. T., Schumacher, A., Grimes, H. 50., et al. (2003). The zinc finger transcription factor Gfi1, implicated in lymphomagenesis, is required for inner ear pilus cell differentiation and survival. Development 130, 221–232. doi: 10.1242/dev.00190
PubMed Abstract | CrossRef Full Text | Google Scholar
Walters, B. J., Coak, Due east., Dearman, J., Bailey, G., Yamashita, T., Kuo, B., et al. (2017). In vivo interplay between p27(Kip1), GATA3, ATOH1 and POU4F3 converts not-sensory cells to hair cells in adult mice. Cell Rep. 19, 307–320. doi: x.1016/j.celrep.2017.03.044
PubMed Abstract | CrossRef Full Text | Google Scholar
Wang, T., Chai, R., Kim, M. S., Pham, N., Jansson, L., Nguyen, D. -H., et al. (2015). Lgr5+ cells regenerate hair cells via proliferation and direct transdifferentiation in damaged neonatal mouse utricle. Nat. Commun. 6:6613. doi: x.1038/ncomms7613
PubMed Abstruse | CrossRef Total Text | Google Scholar
Waqas, K., Zhang, S., He, Z., Tang, M., and Chai, R. (2016). Role of Wnt and Notch signaling in regulating pilus prison cell regeneration in the cochlea. Front. Med. 10, 237–249. doi: x.1007/s11684-016-0464-nine
PubMed Abstract | CrossRef Full Text | Google Scholar
Wu, J., Li, W., Lin, C., Chen, Y., Cheng, C., Dominicus, S., et al. (2016). Co-regulation of the Notch and Wnt signaling pathways promotes supporting cell proliferation and hair jail cell regeneration in mouse utricles. Sci. Rep. half dozen:29418. doi: 10.1038/srep29418
PubMed Abstract | CrossRef Full Text | Google Scholar
Yamamoto, North., Tanigaki, K., Tsuji, M., Yabe, D., Ito, J., and Honjo, T. (2006). Inhibition of Notch/RBP-J signaling induces hair prison cell formation in neonate mouse cochleas. J. Mol. Med. (Berl) 84, 37–45. doi: 10.1007/s00109-005-0706-9
PubMed Abstract | CrossRef Full Text | Google Scholar
Zarei, S., Zarei, Thou., Fritzsch, B., and Elliott, K. L. (2017). Sonic hedgehog antagonists reduce size and alter patterning of the frog inner ear. Dev. Neurobiol. 77, 1385–1400. doi: 10.1002/dneu.22544
PubMed Abstruse | CrossRef Full Text | Google Scholar
Zhang, S., Liu, D., Dong, Y., Zhang, Z., Zhang, Y., Zhou, H., et al. (2019). Frizzled-ix + supporting cells are progenitors for the generation of pilus cells in the postnatal mouse cochlea. Front. Mol. Neurosci. 12:184. doi: 10.3389/fnmol.2019.00184
PubMed Abstract | CrossRef Full Text | Google Scholar
Zhang, S., Zhang, Y., Dong, Y., Guo, 50., Zhang, Z., Shao, B., et al. (2020). Knockdown of Foxg1 in supporting cells increases the trans-differentiation of supporting cells into pilus cells in the neonatal mouse cochlea. Cell. Mol. Life Sci. 77, 1401–1419. doi: x.1007/s00018-019-03291-2
PubMed Abstract | CrossRef Full Text | Google Scholar
Zhang, Y., Zhang, Southward., Zhang, Z., Dong, Y., Ma, Ten., Qiang, R., et al. (2020). Knockdown of Foxg1 in Sox9+ supporting cells increases the trans-differentiation of supporting cells into pilus cells in the neonatal mouse utricle. Aging (Albany NY) 12, 19834–19851. doi: 10.18632/aging.104009
PubMed Abstract | CrossRef Full Text | Google Scholar
Zhong, C., Fu, Y., Pan, Westward., Yu, J., and Wang, J. (2019). Atoh1 and other related central regulators in the development of auditory sensory epithelium in the mammalian inner ear: function and interplay. Dev. Biol. 446, 133–141. doi: 10.1016/j.ydbio.2018.12.025
PubMed Abstract | CrossRef Full Text | Google Scholar
Zinn, E., Pacouret, Due south., Khaychuk, V., Turunen, H. T., Carvalho, L. Due south., Andres-Mateos, Eastward., et al. (2015). In silico reconstruction of the viral evolutionary lineage yields a potent gene therapy vector. Cell Rep. 12, 1056–1068. doi: x.1016/j.celrep.2015.07.019
PubMed Abstract | CrossRef Full Text | Google Scholar
How To Repair Hair Cells In Ear,
Source: https://www.frontiersin.org/articles/10.3389/fncel.2021.732507/full
Posted by: mcalisterandister.blogspot.com


0 Response to "How To Repair Hair Cells In Ear"
Post a Comment